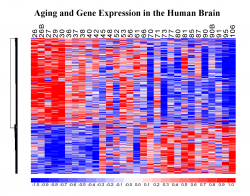
We described the first genome-wide expression profile of the aging human brain, providing evidence for a coherent “signature”of gene expression changes that may underlie brain aging (Lu et al., 2004). By comparing gene expression profiles from multiple species, we determined that the aging process has evolved rapidly in primates and may provide clues to why humans, but not other species, develop age-related brain neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. We are presently investigating the regulation of the aging transcriptome, specifically exploring the role of DNA damage, genetic polymorphisms, somatic mutations and stress response factors. This involves RNA-seq and next generation sequencing in the human brain and in animal models, as well as modeling in primary neuronal cultures and cell lines. By generating age-specific gene expression profiles, we can ask: 1. What are the relationships between epigenetic changes, DNA damage, nuclear factors and the expression of specific genes during aging? And do these relationships change in the genomes of individuals with cognitive decline and Alzheimer’s disease? 2. Why are some individuals able to live past 100 and remain cognitively intact? The molecular basis of extreme longevity and cognitive preservation is poorly understood. To address these intriguing questions, we have taken a multidisciplinary systems biology approach.
Department of Genetics
Research
For Yankner lab publications see: http://www.ncbi.nlm.nih.gov/pubmed/?term=yankner
The aging of the brain is a cause of cognitive decline in the elderly and the major risk factor for Alzheimer's disease (AD). Despite this central role in disease, the molecular underpinnings of brain aging are poorly understood. The overall goal of our research is to achieve a greater understanding of the molecular basis of brain aging and how normal aging transitions to neurodegenerative disorders, particularly Alzheimer’s disease, frontotemporal dementia, Parkinson’s disease, and more recently, chronic traumatic encephalopathy associated with concussion and sports injury. We take diverse approaches to investigate brain aging and disease, with studies ranging from C. elegans to mouse models to informatic analysis of the human brain. This is accompanied by a broad spectrum of collaborative projects (see the lab interactome).
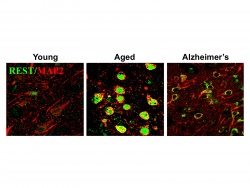
The Aging Brain Needs REST
We have recently discovered that the master developmental regulator REST/NRSF is induced in the aging human brain and coordinates the expression of a gene network that may protect aging neurons from neurotoxic stress, synapse loss and overexcitation (Lu et al., 2014). This pathway regulates the expression of genes that are involved in cell death, inflammation, oxidative stress and AD pathology. Induction of REST correlates with preservation of cognitive function during aging, whereas loss of REST is associated with cognitive decline. Our studies seek to elucidate the regulatory role of the REST network in protecting aging neurons using REST conditional knockout mice and cell culture models. New high-sensitivity transcriptome sequencing technology together with informatics analysis is being used to define the REST-regulated gene network. By applying this systems genetics approach to well-characterized human brain samples from the Religious Orders study, we are defining REST-regulated gene networks predictive of successful aging, early cognitive decline and AD. A central question is how this gene network systematically fails in individuals who develop AD, and whether this decline can be reversed. The protective effects of the REST pathway raises the exciting possibility that the aging brain could be protected by a novel therapeutic approach based on activation of the brain's endogenous defense network.
Genome Instability, Aging and Neurodegenerative Diseases
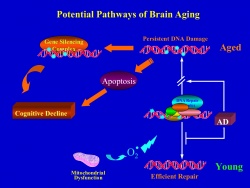
Our lab has shown that the aging of the human brain is associated with DNA damage that may contribute to altered expression of genes involved in synaptic plasticity. We have also found that a particularly severe type of DNA damage, double strand breaks, appears in the brains of AD patients. To increase our understanding of the consequences of genome instability in the brain, we are characterizing a brain-conditional deletion of the XRCC4 gene that mediates DNA repair in the NHEJ pathway. Conditional XRCC4-deficient mice exhibit unrepaired DNA double strand breaks that accumulate predominantly in neurons and increase with age. A second project in collaboration with the Elledge lab at Harvard Medical School focuses on the role of GATA4, which was recently identified as a key senescence-inducing factor in the DNA damage response. GATA4 is unexpectedly induced in the aging brain together with other senescence related factors, raising the possibility that senescence may play a role in the aging of postmitotic neurons as well as replicative cells. Finally, we are embarking on a project to analyze somatic mutations in neurons of the aging brain using single cell next generation sequencing.
Induced Progenitor Stem Cell Models of Aging and Alzheimer’s Disease
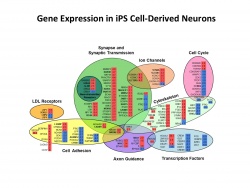
The identification of an expanding number of genetic susceptibility loci for Alzheimer’s disease (AD) provides an opportunity for new mechanistic and therapeutic insights. We have brought together new genome engineering and stem cell technology to further our understanding of AD genetics and pathogenesis. The CRISPR-Cas9 system is a novel and facile platform for genome engineering. In collaboration with George Church’s laboratory, we are using the CRISPR-Cas system to generate a library of isogenic human IPSC lines with AD risk variants in susceptibility genes identified in multi-cohort GWAS studies. We have established IPSC lines from dermal fibroblasts of late-onset AD (LOAD) and age-matched controls that have been differentiated to neurons and astrocytes. These lines, together with the generation of isogenic IPSC lines that differ only in the presence or absence of genetic variants associated with AD, enable us to explore pathogenic mechanisms involving Aβ and tau metabolism, and neuronal stress responses. To elucidate the affected pathways, new high-sensitivity transcriptome sequencing and proteomic technology is being applied to IPSC-derived neurons. The goal of this approach is to identify core regulatory mediators and pathways to provide new insights into the early changes that lead to Alzheimer’s disease.
Modeling aging and neurodegenerative disorders in C. elegans
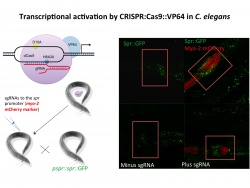
We are studying the role of gene regulation, genome stability and stress resistance in aging and neurodegenerative diseases using the nematode worm C. elegans as a model system. We have shown that worms with mutations in orthologs of the mammalian REST gene exhibit altered stress response phenotypes (Lu et al., 2014). Moreover, many of these phenotypic profiles are conserved from worms to humans. We are using this model system to define the signaling pathways that regulate these phenotypes. In collaboration with Monica Colaiacovo’s lab, we are also investigating the role of genome stability in C. elegans aging and stress resistance. In collaboration with George Church’s lab, we have developed a variation of the CRISPR-CAS9 system in which a CAS9-VP64 fusion protein can be targeted by small guide RNAs to any promoter to transactivate endogenous genes. This approach is being used to simultaneously activate multiple members of a transcription factor/epigenetic complex to achieve physiological regulation of gene expression. As such, we use the worm as a model to test hypotheses and expand our understanding of aging biology and neurodegenerative diseases.
Novel therapeutic approaches to Alzheimer’s disease and brain aging
Our lab has historically been involved in defining the underlying neurodegenerative mechanisms in Alzheimer’s disease and in the search for therapeutic agents. We discovered the phenomenon of amyloid neurotoxicity and identified the first anti-amyloid aggregation agents. Our present approach, however, seeks to mimic mechanisms of successful aging in humans by increasing our understanding of how some people can live long lives without cognitive problems. The goal is to recapitulate the molecular mechanisms of successful aging with therapeutic agents. To accomplish this goal, we perform genomic screens in worms and neuronal cells in culture, and then investigate potential therapeutic targets in animal models and in the human brain.
Copyright © The President and Fellows of Harvard College