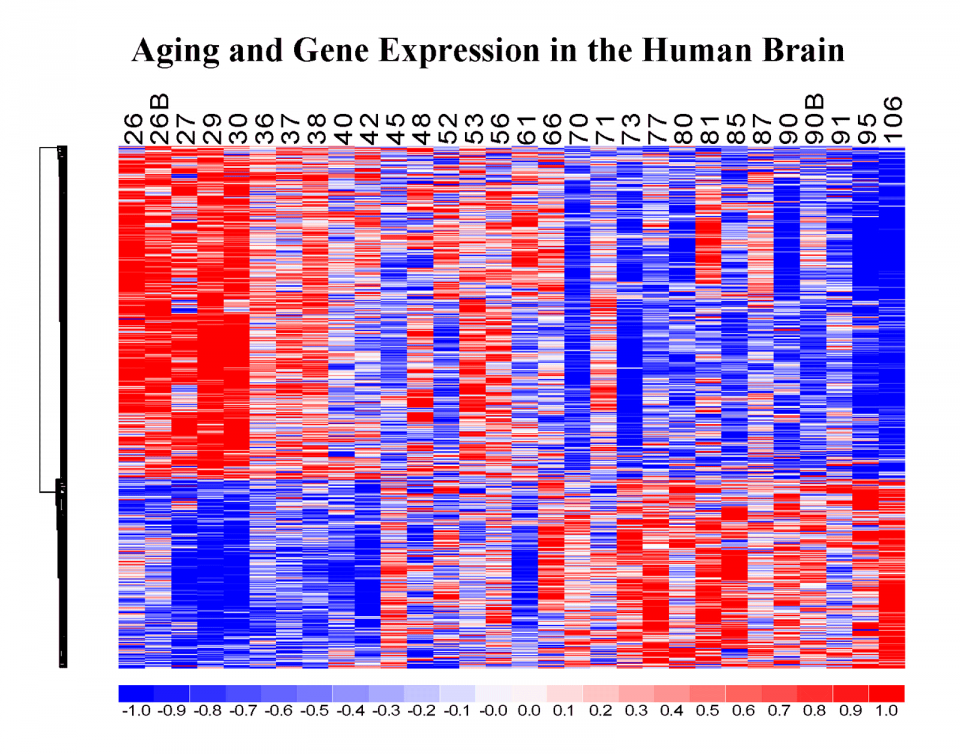
We described the first genome-wide expression profile of the aging human brain, providing evidence for a coherent “signature”of gene expression changes that may underlie brain aging (Lu et al., 2004). By comparing gene expression profiles from multiple species, we determined that the aging process has evolved rapidly in primates and may provide clues to why humans, but not other species, develop age-related brain neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. We are presently investigating the regulation of the aging transcriptome, specifically exploring the role of DNA damage, genetic polymorphisms, somatic mutations and stress response factors. This involves RNA-seq and next generation sequencing in the human brain and in animal models, as well as modeling in primary neuronal cultures and cell lines. By generating age-specific gene expression profiles, we can ask: 1. What are the relationships between epigenetic changes, DNA damage, nuclear factors and the expression of specific genes during aging? And do these relationships change in the genomes of individuals with cognitive decline and Alzheimer’s disease? 2. Why are some individuals able to live past 100 and remain cognitively intact? The molecular basis of extreme longevity and cognitive preservation is poorly understood. To address these intriguing questions, we have taken a multidisciplinary systems biology approach.
Blavatnik Institute
Department of Genetics
Department of Genetics
Transcriptional and Epigenetic Regulation in the Aging Brain
Copyright © The President and Fellows of Harvard College