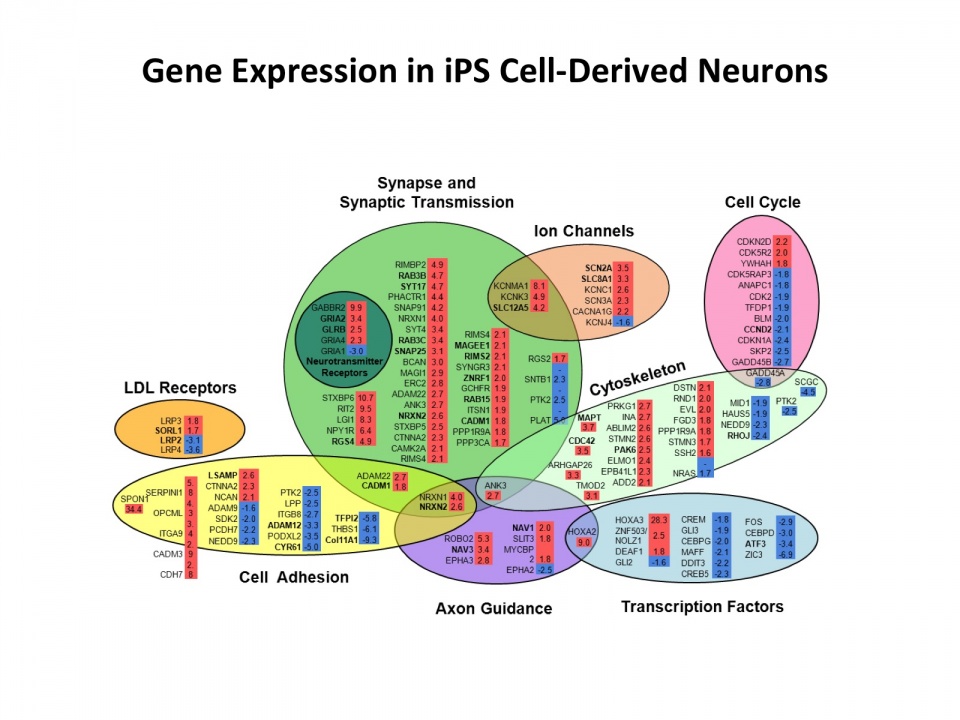
The identification of an expanding number of genetic susceptibility loci for Alzheimer’s disease (AD) provides an opportunity for new mechanistic and therapeutic insights. We have brought together new genome engineering and stem cell technology to further our understanding of AD genetics and pathogenesis. The CRISPR-Cas9 system is a novel and facile platform for genome engineering. In collaboration with George Church’s laboratory, we are using the CRISPR-Cas system to generate a library of isogenic human IPSC lines with AD risk variants in susceptibility genes identified in multi-cohort GWAS studies. We have established IPSC lines from dermal fibroblasts of late-onset AD (LOAD) and age-matched controls that have been differentiated to neurons and astrocytes. These lines, together with the generation of isogenic IPSC lines that differ only in the presence or absence of genetic variants associated with AD, enable us to explore pathogenic mechanisms involving Aβ and tau metabolism, and neuronal stress responses. To elucidate the affected pathways, new high-sensitivity transcriptome sequencing and proteomic technology is being applied to IPSC-derived neurons. The goal of this approach is to identify core regulatory mediators and pathways to provide new insights into the early changes that lead to Alzheimer’s disease.
Blavatnik Institute
Department of Genetics
Department of Genetics
Induced Progenitor Stem Cell Models of Aging and Alzheimer’s Disease
Copyright © The President and Fellows of Harvard College